A recent open-label study in patients with pulmonary sequelae subsequent to COVID-19 infection indicated
that Longidaza® was associated with improvements in pulmonary function, exercise tolerance,
and
respiratory symptoms7.
We conducted the “Long-CoV-III” randomized, double-blind, placebo-controlled clinical trial to further
evaluate the possible effects of Longidaza® on COVID-19 pulmonary sequelae.
Long-CoV-III was a randomized, double-blind, placebo-controlled trial in patients with a history of
COVID-19, residual lung abnormalities, restrictive pulmonary disease, dyspnea, and decreased oxygen
saturation at rest or after exercise. The study was conducted at 37 sites. A total of 392 patients were
randomized 1:1 to receive intramuscular injections of Longidaza® (3000 IU) or placebo every 5
days for
71 days. After completion of treatment, patients entered a 109-day follow-up. The efficacy was assessed
in the full analysis set of 376 patients who successfully completed the study.
In total, 392 patients were randomized and dosed with Longidaza® or placebo; 382 completed
the trial.
All randomized patients were included in the safety analysis, while the Full Analysis Set (FAS)
comprised 376 patients (186 Longidaza®, 190 placebo). Patients were excluded from the FAS
primarily due
to non-compliance with restrictive pulmonary disease criteria (FVC<80%, FEV1/FVC>70%).
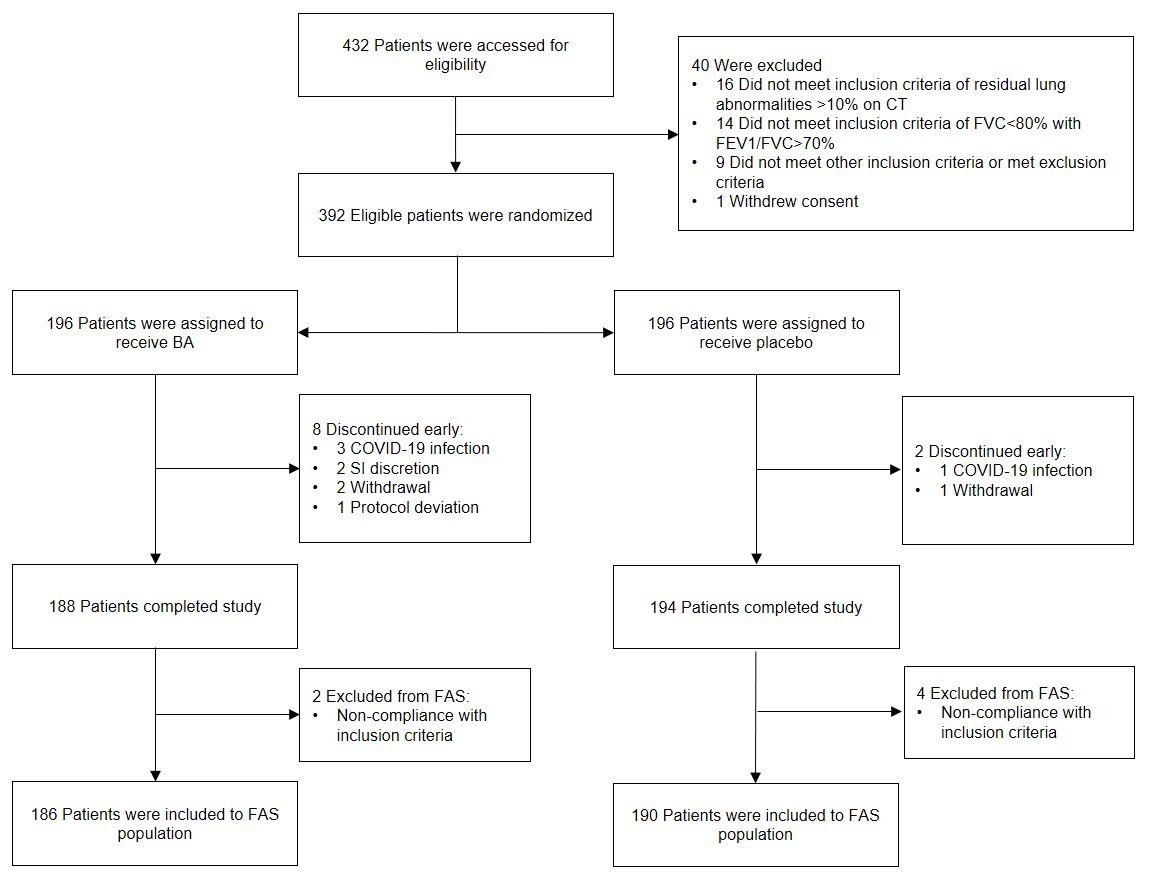
*CONSORT flow diagram. BA: Longidaza; FAS: full analysis set; SI: site investigator.
Primary endpoint:
-
Change from baseline in Forced Vital Capacity (percent of predicted, ppFVC) by Day 71
Secondary endpoints related to pulmonary function:
-
Change from baseline in ppFVC by Day 180
-
The proportion of patients who achieved at least 10% ppFVC increase by days 71 and 180.
Secondary endpoint related to exercise tolerance and respiratory symptoms:
-
The proportion of patients exhibiting exertional desaturation (defined as a ≥4% decrease in
SpO2 after 6-minute walk test (6-MWT)) on days 71 and 180
-
The proportion of patients with exertional dyspnea (defined by a ≥2 point increase on the Borg
scale after 6-MWT) on days 71 and 180
-
The proportion of patients achieving a ≥50 meter increase in distance walked during the 6-MWT
compared to the distance at baseline on days 71 and 180
-
The proportion of patients with a decrease in resting dyspnea from baseline by ≥1 point on the
mMRC scale on days 71 and 180
For exploratory subgroup analysis, patients were stratified by COVID-19 start date (before February 14,
2022, vs. on or after February 14, 2022); by age (≤60 years vs. >60 years); by sex; by the presence of
cardiovascular comorbidities defined by MedDRA system organ classes.
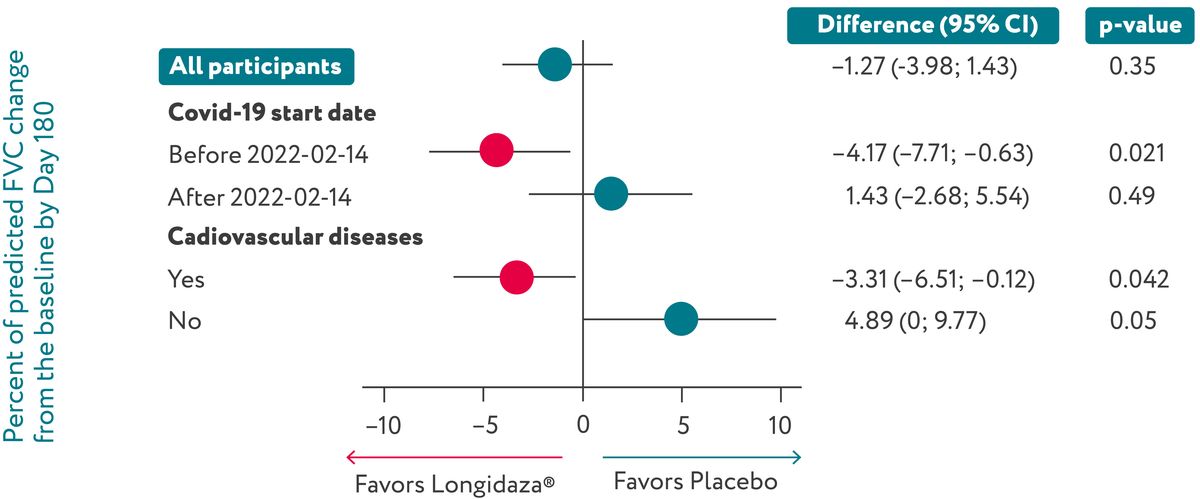
In the overall population Longidaza® had no significant
effect on
ppFVC
growth. However, it was associated with faster ppFVC recovery in patients with cardiovascular
comorbidities (n=281, diff=-3.31%, p=0.042) and those with an earlier COVID-19 infection (n=188,
diff=-4.17%, p=0.021).
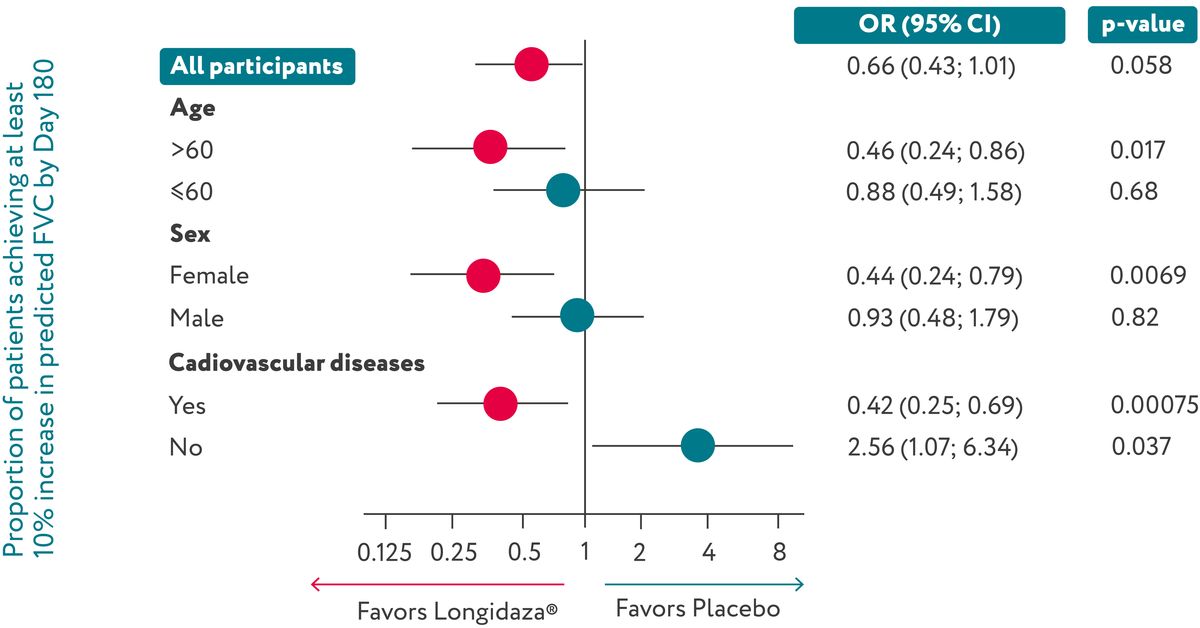
Longidaza® showed a trend towards an increase in the proportion of patients achieving ≥10%
ppFVC growth.
On Day 180, 68% of patients in the Longidaza® group and 58.4% of patients in the placebo
group exhibited at
least a 10% ppFVC increase from baseline (OR=1.51 ; p=0.058). Longidaza® was also linked
to a
significantly higher proportion of patients in specific subgroups (age >60, female, and with
cardiovascular comorbidities) achieving a 10% increase in ppFVC by Day 180.
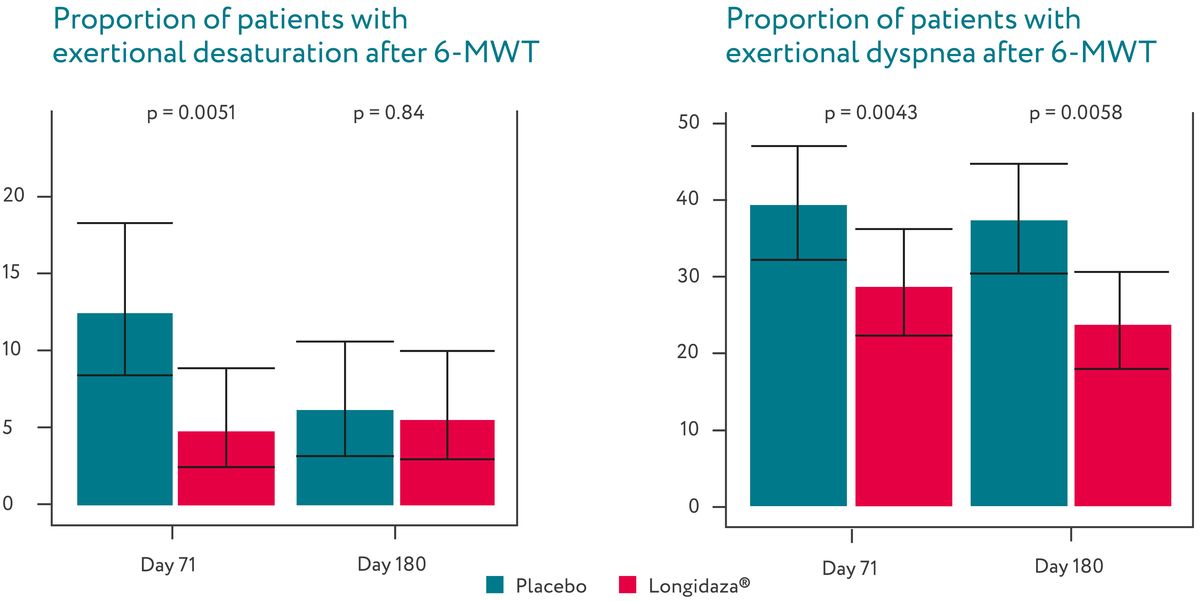
Longidaza® was associated with a significant decrease in the proportion of patients
experiencing
desaturation and dyspnea after a six-minute walk test (6-MWT) at days 71 and 180.
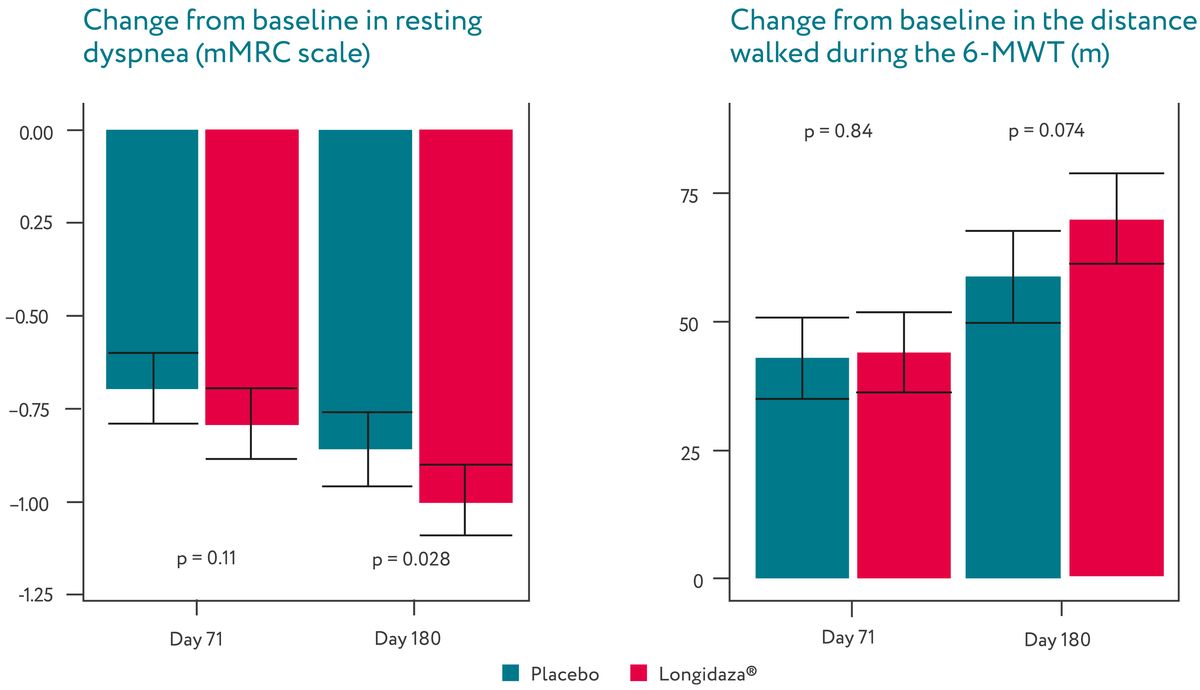
Longidaza® was associated with a significant decrease in resting dyspnea and a trend towards
the
increased distance walked in six-minute test by Day 180.
Seventy-three (37%) of 196 patients in the Longidaza® group and 49 (25%) of 196 patients in
the placebo
group experienced adverse events during the study. Eight adverse events in seven patients were deemed
related to Longidaza®. These included three injection site reactions, and single cases of
elevated
alanine aminotransferase levels, elevated C-reactive protein, thrombocytopenia, hives, and angina
pectoris. One participant receiving placebo experienced a case of thrombocytopenia that was considered
related to treatment. Three patients in the Longidaza® group had serious adverse events,
though these were not
related to Longidaza®. Adverse events led to the discontinuation of the study drug in five
patients receiving Longidaza®
and one patient in the placebo group.
|
Event
|
Longidaza
|
Placebo
|
|
|
number of patients (%)
|
| Any adverse events
|
73 (37.2)
|
49 (25)
|
| Most frequent adverse events
|
|
|
| - Respiratory tract infection
|
11 (5.6)
|
13 (6.6)
|
| - COVID-19
|
4 (2)
|
-
|
| Severe adverse event
|
3 (1.5)
|
-
|
| - Gastroenteritis
|
1 (0.5)
|
-
|
| - COVID-19
|
1 (0.5)
|
-
|
| - Cholecystitis
|
1 (0.5)
|
-
|
| Adverse event leading to discontinuation of study treatment
|
5 (2.5)
|
1 (0.5)
|
| - COVID-19
|
3 (1.5)
|
1 (0.5)
|
| - Candidiasis
|
1 (0.5)
|
-
|
| - Gastrointestinal diseases
|
1 (0.5)
|
-
|
| Adverse event considered related to study drug
|
7 (3.6)
|
1 (0.5)
|
| - Injection reaction in 3 patients and hives in 1 patient
|
3 (1.5)
|
-
|
| - Elevated C-reactive protein
|
1 (0.5)
|
-
|
| - Elevated alanine aminotransferase
|
1 (0.5)
|
-
|
| - Thrombocytopenia
|
1 (0.5)
|
1 (0.5)
|
| - Angina pectoris
|
1 (0.5)
|
-
|

Avdeev, Sergey N., et al. "Bovhyaluronidase azoximer for long-term pulmonary sequelae of COVID-19:
a
randomized, double-blind, placebo-controlled trial." medRxiv (2024): 2024-09.